Cell & gene therapy applications
Elevate your advanced therapy with bespoke promoter solutions
The importance of promoters for gene and cell therapies
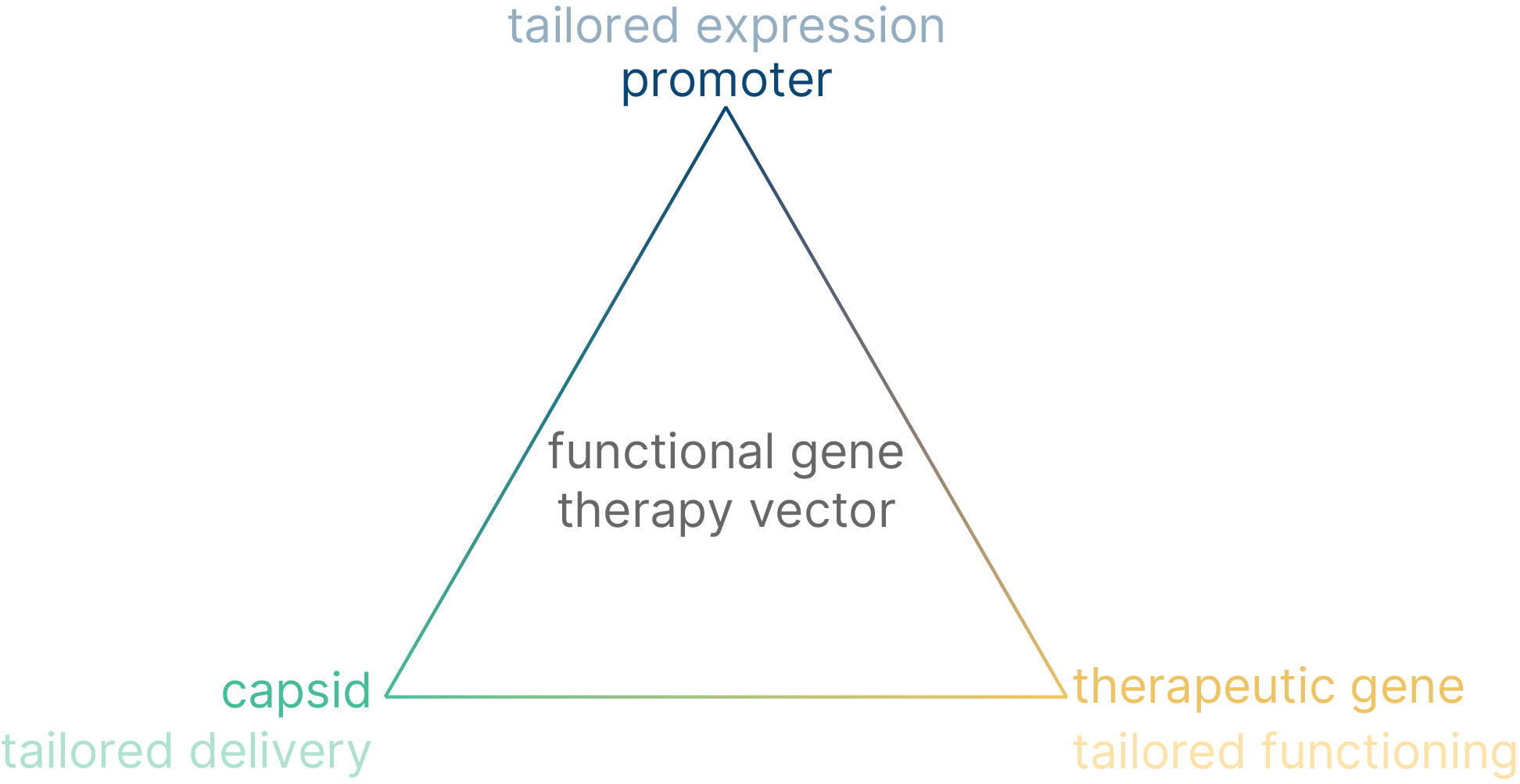
The safety and effectiveness of cell and gene therapies depend on getting the therapeutic transgene to express at the right level, in the right cells and at the right moment.
To improve these aspects, manufacturers can adjust three principal components: the therapeutic gene, the viral vector (including the viral capsid) and the promoter.
Speak to an expert →
To achieve cell type specific transgene expression, efforts have historically focused on capsid engineering. However, therapy-tailored promoters can define cell type specific expression as well and, moreover, can dictate the expression level and the moment of expression, which is something that capsids cannot provide.
At Annogen, we partner with leading academics, biopharmaceutical manufacturers and consortia to help develop the next generation of advanced therapies. In every collaboration we focus on what we know best: how to engineer the most effective gene expression system.
Bespoke promoter identification for advanced therapies
Annogen’s SuRE™ screen exhaustively screens for the regulatory activity of millions of genomic elements to select a combination of human DNA elements with the optimal expression profile for a specific therapy. We have experience developing promoters for specific expression levels, tissue types, conditions and stimuli. Bespoke promoters allow for lasting therapeutic effects, targeted specifically to the cell type where treatment is needed, while minimizing adverse effects in off-target tissues such as the liver and dorsal root ganglia (DRG). Customers for which we execute a bespoke screening program, typically obtain exclusive rights to the identified top performing promoters.
Which gene promoters would elevate your therapies to the next level?
We believe that nature knows best. Therefore, in contrast to in silico design or fully synthetic sequences, Annogen screens existing DNA elements and combinations of these to identify the optimal promoter design. Therefore, our promoters for advanced therapies are of human origin and validated before being delivered to our customers.
Learn more about SuRE™ →

Join other trusted biopharmaceutical developers including Pfizer, Orchard Therapeutics, uniQure, Meira GTx, Novo Nordisk and VectorY in designing the next generation of advanced therapies together with Annogen.
In vivo validations
Annogen can perform in vivo AAV-SuRE™ promoter screening to ensure testing under conditions as close as possible to the in vivo application. Upon customer requests, SuRE™ libraries can be administered and screened in mice, pigs or NHPs. This approach reduces the number of animals used in research by generating more comprehensive data in a single experiment, limiting the need for testing candidate promoters in separate animals.
Promoter optimization for advanced therapies
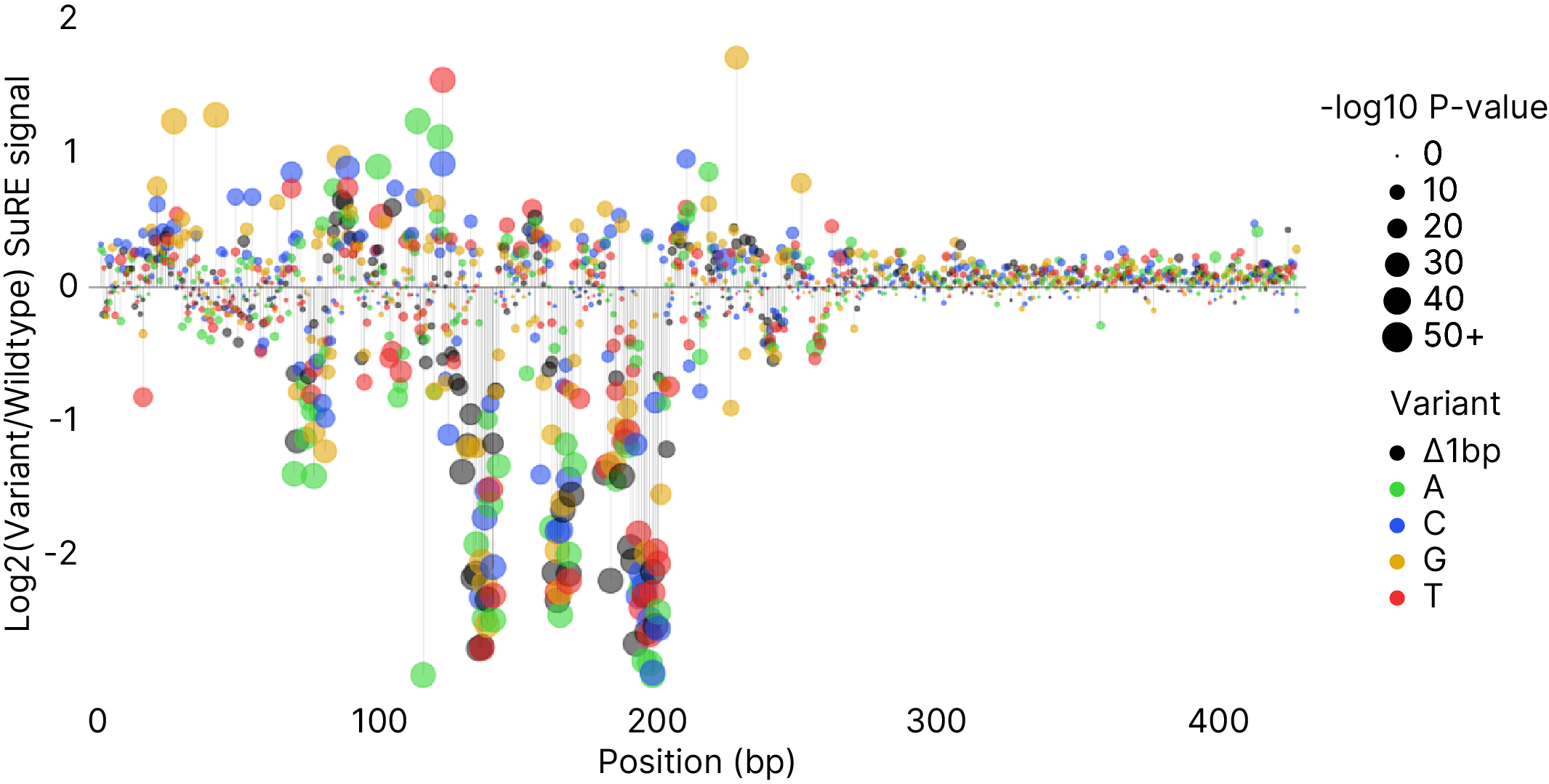
In addition to bespoke promoter identification screens, SuRE™ can also be used to optimize existing promoters. As the basis for such a optimization project we perform a saturation mutagenesis (SatMut), coupled to our SuRE™ readout, which shows the impact of each possible single base mutation on gene expression.
The results of such a SatMut analysis can form the basis for new promoter designs focused for example on increasing promoter activity, reducing background activity, CpG removal or promoter minimization without the loss of expression.
Typically a SuRE™ mutagenesis screen takes about 3 months and can also be performed for several regions in parallel, depending on the end goal of the customer.
Off-the-shelf promoters for advanced therapies
Besides bespoke screening programs for our customers, our scientists are continuously optimizing our platforms and performing in-house designs of powerful promoters for use in advanced therapy products.
Currently we have the following promoters that are readily available for licensing for applications in cell and gene therapy:
hCon: human strong constitutive promoter (size ~700bp)
Annogen’s human constitutive (hCon) promoter is a human genome-derived proprietary promoter with a similar expression levels to commonly used viral promoters such as CBh and CAG, but showing more stability during virus production, more homogenous expression levels and no stress related induction as seen for CBh and CAG. This promoter has been validated in NHP and is expected to be used in human for the first time Q4 2025. Annogen also has smaller hCon promoters of ~300bp under development for applications in which there is limited space available in the vector.
hITA: human promoter induced upon T-cell activation (size ~1000 bp)
The commonly used NFAT-promoter in the cell therapy field is considered leaky and suboptimal and there is a broad interest in replacing it. Our hITA promoter is inducible at T-cell activation and shows a more than 100-fold induction. This promoter has been validated in Jurkat cells and primary T-cells where we see substantially higher proportions of cells expressing our transgene under the hITA promoter, at higher expression levels and at lower antigen levels.
hIN: human promoter induced upon neuroinflammation (size ~1200 bp)
The hIN promoter is a human genome derived proprietary SuRE™ element inducible at neuroinflammation that shows a more than 30-fold induction. This promoter has been validated in an in vivo MS model showing strong induction upon inflammation in the spinal cord.
AIM expression locus analysis for advanced therapies
Our AIM™ platform allows for the identification of gene insertions and their expression levels for 100,000 integrations in parallel.
Controlled transgene expression is fundamental for safe and effective cell therapies. Transgenes in these therapies are often integrated at random genomic locations, for example with lentivirus or transposases, because these systems are very efficient. However, such random integrations may also result in clonal expansion, oncogenic transformation, variegated transgene expression and transcriptional silencing. Therefore, targeted integration into a predefined locus is an attractive alternative to random integration and is increasingly used in cell therapies. However, the number of known loci that facilitate correct gene expression (e.g AAVS1, TRAC) is limited and based on decades of rational design-based research.
Annogen’s AIM™ platform enables the systematic characterization of the positional effect of integration sites on transgene expression, for 100,000 integrations in parallel. AIM™ thus greatly speeds up the identification of novel integration sites for cell therapy. AIMTM studies can be performed in a tailored manner to yield patentable loci that allow for the best possible therapeutic effect.
To learn more about the added value of AIM™ to your work, check out our poster on the use of AIM™ to identify genomic loci that are induced upon T-cell activation.
Annogen’s AIM™ screen: bespoke expression locus identification for more effective cell therapies
Annogen’s AIM™ screen revolutionizes the level of control researchers have over transgene expression by identifying optimal genomic loci for transgene integration. Choosing the integration site with the right expression profile will enable safer and more effective cell and gene therapies.